In patient-physician
contacts, decision-making is a critical activity, with judgements often based
on partial and insufficient patient information.
In principle, physician decision-making, which is undeniably
complicated and dynamic, is hypothesis-driven.
Diagnostic intervention is based on a hypothetically
deductive process of testing hypotheses against clinical evidence to arrive at
conclusions.
Evidence-based medicine is a method of medical practice that
incorporates individual clinical skill and experience with the best available
external evidence from scientific literature to enhance decision-making.
Evidence-based medicine must be based on the highest
quality, most trustworthy, and systematic data available.
The important issues remain, knowing that both
evidence-based medicine and clinical research are required, but that none is
perfect: How can doctors get the most up-to-date scientific evidence? What
constitutes the best evidence? How may doctors be helped to decide whether
external clinical evidence from systematic research should have an impact on
their practice? A hierarchy of evidence may help you figure out which sorts of
evidence are more likely to produce reliable answers to clinical problems if
done correctly.
Despite the lack of a broadly agreed hierarchy of evidence, Alba DiCenso et al. (2009) established the 6S Hierarchy of Evidence-Based Resources as a framework for classifying and selecting resources that assess and synthesize research results.
The 6S pyramid was created to help doctors and other
health-care professionals make choices based on the best available research
data.
It shows a hierarchy of evidence in which higher levels give
more accurate and efficient forms of information.
Individual studies are at the bottom of the pyramid.
Although they serve as the foundation for research, a single
study has limited practical relevance for practicing doctors.
Clinicians have been taught for years that randomized
controlled trials are the gold standard for making therapeutic decisions.
Researchers may use randomized controlled trials to see
whether a treatment or intervention is helpful in a particular patient
population, and a strong randomized controlled trial can overturn years of
conventional wisdom.
Physicians, on the other hand, care more about whether it
will work for their patient in a specific situation.
A randomized controlled study cannot provide this
information.
A research synthesis may be considered of as a study of
studies, since it reflects a greater degree of evidence than individual
studies.
It makes conclusions about a practice's efficacy by
carefully examining evidence from various experimental investigations.
Systematic reviews and meta-analyses, which are often seen
as the pillars of evidence-based medicine, have their own set of issues and
rely on rigorous evaluation of the features of the available data.
The problem is that most doctors are unfamiliar with the
statistical procedures used in a meta-analysis and are uncomfortable with the
fundamental scientific ideas needed to evaluate data.
Clinical practice recommendations are intended to bridge the
gap between research and existing practice, reducing unnecessary variation in
practice.
In recent years, the number of clinical practice
recommendations has exploded.
The development process is largely responsible for the
guidelines' credibility.
The most serious problem is the lack of scientific evidence
that these clinical practice guidelines are based on.
They don't all have the same level of quality and
trustworthiness in their evidence.
The search for evidence-based resources should start at the
top of the 6S pyramid, at the systems layer, which includes computerized
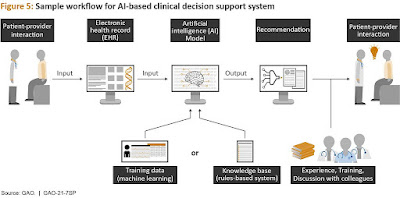
clinical decision support systems.
Computerized clinical decision support systems (also known
as intelligent medical platforms) are health information technology-based
software that builds on the foundation of an electronic health record to
provide clinicians with intelligently filtered and organized general and
patient-specific information to improve health and clinical care.
Laboratory measurements, for example, are often color-coded
to show whether they lie inside or outside of a reference range.
The computerized clinical decision support systems that are
now available are not a simple model that produces just an output.
Multiple phases are involved in the interpretation and use
of a computerized clinical decision support system, including displaying the
algorithm output in a specified fashion, the clinician's interpretation, and
finally the medical decision.
Despite the fact that computerized clinical decision support
systems have been proved to minimize medical mistakes and enhance patient outcomes,
user acceptability has prevented them from reaching their full potential.
Aside from the interface problems, doctors are wary about
computerized clinical decision support systems because they may limit their
professional autonomy or be utilized in the case of a medical-legal dispute.
Although computerized clinical decision support systems
still need human participation, some critical sectors of medicine, such as
cancer, cardiology, and neurology, are adopting artificial intelligence-based
diagnostic tools.
Machine learning methods and natural language processing
systems are the two main groups of these instruments.
Patients' data is used to construct a structured database
for genetic, imaging, and electrophysiological records, which is then analyzed
for a diagnosis using machine learning methods.
To assist the machine learning process, natural language
processing systems construct a structured database utilizing clinical notes and
medical periodicals.
Furthermore, machine learning algorithms in medical
applications seek to cluster patients' features in order to predict the
likelihood of illness outcomes and offer a prognosis to the clinician.
Several machine learning and natural language processing
technologies have been coupled to produce powerful computerized clinical
decision support systems that can process and offer diagnoses as well as or
better than doctors.
When it came to detecting lymph node metastases, a
Google-developed AI approach called convolutional neural networking surpassed
pathologists.
In compared to pathologists, who had a sensitivity of 73
percent, the convolutional neural network was sensitive 97 percent of the time.
Furthermore, when the same convolutional neural network was
used to classify skin cancers, it performed at a level comparable to
dermatologists (Krittanawong 2018).
Depression is also diagnosed and classified using such
approaches.
By merging artificial intelligence's capability with human
views, empathy, and experience, physicians' potential will be increased.
The advantages of advanced computerized clinical decision
support systems, on the other hand, are not limited to diagnoses and
classification.
By reducing processing time and thus improving patient care,
computerized clinical decision support systems can be used to improve
communication between physicians and patients.
To avoid drug-drug interactions, computerized clinical
decision support systems can prioritize medication prescription for patients
based on their medical history.
More importantly, by extracting past medical history and
using patient symptoms to determine whether the patient should be referred to
urgent care, a specialist, or a primary care doctor, computerized clinical
decision support systems equipped with artificial intelligence can aid triage
diagnosis and reduce triage processing times.
Because they are the primary causes of mortality in North
America, developing artificial intelligence around these acute and highly
specialized medical problems is critical.
Artificial intelligence has also been used in other ways
with computerized clinical decision support systems.
The studies of Long et al. (2017), who used ocular imaging data to identify congenital cataract illness, and Gulshan et al.
(2016), who used retinal fundus pictures to detect referable
diabetic retinopathy, are two recent instances.
Both stories show how artificial intelligence is growing
exponentially in the medical industry and how it may be used in a variety of
ways.
Although computerized clinical decision support systems hold
great promise for facilitating evidence-based medicine, much work has to be
done to reach their full potential in health care.
The growing familiarity of new generations of doctors with
sophisticated digital technology may encourage the usage and integration of
computerized clinical decision support systems.
Over the next decade, the market for such systems is
expected to expand dramatically.
The pressing need to lower the prevalence of drug mistakes
and worldwide health-care expenditures is driving this expansion.
Computerized clinical decision support systems are the gold
standard for assisting and supporting physicians in their decision-making.
In order to benefit doctors, patients, health-care
organizations, and society, the future should include more advanced analytics,
automation, and a more tailored interaction with the electronic health record.
~ Jai Krishna Ponnappan
You may also want to read more about Artificial Intelligence here.
See also:
Automated Multiphasic Health Testing; Expert Systems; Explainable AI; INTERNIST-I and QMR.
Further Reading
Arnaert, Antonia, and Norma Ponzoni. 2016. “Promoting Clinical Reasoning Among Nursing Students: Why Aren’t Clinical Decision Support Systems a Popular Option?” Canadian Journal of Nursing Research 48, no. 2: 33–34.
Arnaert, Antonia, Norma Ponzoni, John A. Liebert, and Zoumanan Debe. 2017. “Transformative Technology: What Accounts for the Limited Use of Clinical Decision Support Systems in Nursing Practice?” In Health Professionals’ Education in the Age of Clinical Information Systems, Mobile Computing, and Social Media, edited by Aviv Shachak, Elizabeth M. Borycki, and Shmuel P. Reis, 131–45. Cambridge, MA: Academic Press.
DiCenso, Alba, Liz Bayley, and R. Brian Haynes. 2009. “Accessing Preappraised Evidence: Fine-tuning the 5S Model into a 6S Model.” ACP Journal Club 151, no. 6 (September): JC3-2–JC3-3.
Gulshan, Varun, et al. 2016. “Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs.” JAMA 316, no. 22 (December): 2402–10.
Krittanawong, Chayakrit. 2018. “The Rise of Artificial Intelligence and the Uncertain Future for Physicians.” European Journal of Internal Medicine 48 (February): e13–e14.
Long, Erping, et al. 2017. “An Artificial Intelligence Platform for the Multihospital Collaborative Management of Congenital Cataracts.” Nature Biomedical Engineering 1, no. 2: n.p.
Miller, D. Douglas, and Eric W. Brown. 2018. “Artificial Intelligence in Medical Practice: The Question to the Answer?” American Journal of Medicine 131, no. 2: 129–33.